1.800.858.7378npic@oregonstate.edu
Call, email, or chat Mon-Fri
A to Z
2,4-D Technical Fact Sheet
As of 2011, NPIC stopped creating technical pesticide fact sheets. The old collection of technical fact sheets will remain available in this archive, but they may contain out-of-date material. NPIC no longer has the capacity to consistently update them. To visit our general fact sheets, click here. For up-to-date technical fact sheets, please visit the Environmental Protection Agency's webpage.
- Chemical Class and Type
- Physical / Chemical Properties
- Uses
- Mode of Action
- Toxicity Classification
- Acute Toxicity
- Chronic Toxicity
- Endocrine Disruption
- Carcinogenicity
- Reproductive and Teratogenic Effects
- Fate in the Body
- Medical Tests and Monitoring
- Environmental Fate
- Ecotoxicity Studies
- Regulatory Guidelines
Chemical Class and Type:
- 2,4-D is an herbicide and secondarily a plant growth regulator.1 Formulations include esters, acids, and several salts, which vary in their chemical properties, environmental behavior, and to a lesser extent, toxicity.2,3 The salt and ester forms are derivatives of the parent acid.2 Unless otherwise stated, the discussion in this fact sheet will refer to the acid form.
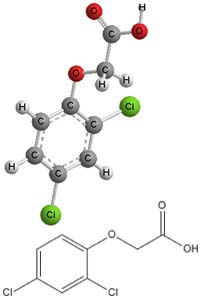
- The International Union of Pure and Applied Chemistry (IUPAC) chemical name for the acid form is 2,4-dichlorophenoxyacetic acid, its Chemical Abstracts Service (CAS) registry number is 94-75-7, and the chemical family is the phenoxyacetic acid compounds.3
- The dimethyl-amine salt (DMA) and 2-ethylhexyl ester (EHE) forms account for approximately 90-95% of the total global use.4 The acid form is low in solubility and herbicide formulations consist of more soluble forms of the chemical.2 Products containing 2,4-D frequently contain other herbicides as well.5
- Agent Orange, the herbicide widely used during the Vietnam war, contained 2,4-D. However, the controversy regarding health effects centered around the 2,4,5-T component of the herbicide and its contaminant, dioxin.6,7
- 2,4-D has been used in the United States since the 1940s, and it was evaluated for re-registration in 2005 by the United States Environmental Protection Agency (U.S. EPA).3 The U.S. EPA determined that 2,4-D was eligible for re-registration, but required certain changes to labeled uses to mitigate risk.3 See the text box on Laboratory Testing.
Molecular Structure - 2,4-D
Laboratory Testing: Before pesticides are registered by the U.S. EPA, they must undergo laboratory testing for short-term (acute) and long-term (chronic) health effects. Laboratory animals are purposely given high enough doses to cause toxic effects. These tests help scientists judge how these chemicals might affect humans, domestic animals, and wildlife in cases of overexposure.
| 2,4-D and associated forms8,9 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Active Ingredient | CASRN | Form | Vapor pressurea | Henry's constant | Molecular weight | Solubility in water (mg/L)b | Log Kow | Koc |
| 2,4-D acid | 94-75-7 | White to brown crystalline solid | 1.9 x 10-5 Pa 1.4 x 10-7 mmHg |
8.6 x 10-6 atm·m3/mol | 221 | pH 5: 29,934 ± 2957b
pH 7: 44,558 ± 674 pH 9: 43,134 ± 336 |
0.001 M sol'n
pH 5: 2.14 pH 7: 0.177 pH 9: 0.102 |
20-136 |
| 2,4-D salt | 2702-72-9 | White powder | Salt dissociates to acid in water | 243.03 | 45,000 mg/L | Salt dissociates to acid in water | ||
| 2,4-D-diethanolamine salt (DEA) | 5742-19-8 | Cream colored powder | 9.98 x 10-8 mmHg | 326.18 | 806,000 mg/L | 2.24 x 10-2 -1.65 |
||
| 2,4-D dimethyl amine salt (DMA) | 2008-39-1 | Amber aqueous liquid | 1.33 x 10-5 Pa
1 x 10-7 mmHg |
1.4 x 10-16 atm·m3/mol | 266.13 | pH 5: 320,632 ± 3645
pH 7: 729,397 ± 86,400 pH 9: 663,755 ± 94,647 |
See values for 2,4-D acid above | 72-136 |
| 2,4-D -isopropylamine (IPA) salt | 5742-17-6 | Amber aqueous liquid | Salt dissociates to acid in water | 280.04 | pH 5: 174,000 mg/L
pH 7: 436,000 mg/L pH 9: 331,000 mg/L |
Salt dissociates to acid in water | ||
| 2,4-D tri-isopropanolamine (TIPA) salt | 32341-80-3 | Amber aqueous liquid | Salt dissociates to acid in water | 412.31 | pH 5: 461,000 mg/L
pH 7: 461,000 mg/L pH 9: 104,000 mg/L |
Salt dissociates to acid in water | ||
| 2,4-D BEE | 1929-73-3 | Dark amber liquid | 3.2 x 10-4 Pa
2.4 x 10-6 mmHg |
321.2 | Practically insoluble in water | 4.1 | ||
| 2,4-D 2-ethylhexyl ester (EHE) | 1928-43-4 | Dark amber liquid | 4.8 x 10-4 Pa
3.6 x 10-6 mmHg |
333.27 | 0.0867 mg/L | 5.78 | ||
| 2,4-D -isopropyl ester (IPE) | 94-11-1 | Pale amber liquid | 1.87 Pa
5.3 x 10-6 mbar |
2.2 x 10-6 atm·m3/mol | 263.12 | Practically insoluble in water | 253.8 ± 44.4 | 600 |
| aVapor pressure measured at 25 °C | bSolubility in water given for unbuffered solution | |||||||
Uses:
- 2,4-D is used for broadleaf weed control in agricultural and nonagricultural settings, and it is registered for use in both terrestrial and aquatic environments. Major sites include pasture and rangeland, residential lawns, roadways, and cropland. Crops treated with 2,4-D include field corn, soybeans, spring wheat, hazelnuts, sugarcane, and barley.3 Uses for products containing 2,4-D vary widely. Always read and follow the label when applying pesticide products.
- Approximately 46 million pounds are used each year in the United States, based on data from 1992-2000.3
- Signal words for products containing 2,4-D may range from Caution to Danger.10 The signal word reflects the combined toxicity of the active ingredient and other ingredients in the product. See the pesticide label on the product and refer to the NPIC fact sheets on Signal Words and Inert or "Other" Ingredients.
- To find a list of products containing 2,4-D which are registered in your state, visit the website https://npic.orst.edu/reg/state_agencies.html select your state then click on the link for "State Products."
Mode of Action:
Target Organisms
- 2,4-D is used on a wide variety of terrestrial and aquatic broadleaf weeds. It has little effect on grasses.12 It appears to work by causing uncontrolled cell division in vascular tissue.12 Abnormal increases in cell wall plasticity, biosynthesis of proteins, and production of ethylene occur in plant tissues following exposure, and these processes are responsible for uncontrolled cell division.3,12
- The ester forms of 2,4-D penetrate foliage, whereas plant roots absorb the salt forms.12 2,4-D appears to be similar in action to other auxin-type herbicides.12
Non-target Organisms
- The modes of toxicity to animals from the acid, ester and salt forms of 2,4-D are similar. The primary exception is that the salt and acid forms can be extreme eye irritants.3 2,4-D is actively secreted by the proximal tubules of the kidney, and toxicity appears to result when renal clearance capacity is exceeded.3 Dose-dependent toxic effects include damage to the eye, thyroid, kidney, adrenals, and ovaries or testes.3 In addition, researchers have observed neurotoxicity, reproductive toxicity, and developmental toxicity.3 Chlorophenoxy herbicides exhibit a variety of mechanisms of toxicity, including dose-dependent cell membrane damage leading to central nervous system toxicity,13 interference with cellular metabolism involving acetyl-coenzyme A (CoA),13 and uncoupling of oxidative phosphorylation due to either the disrupted CoA activity or cellular membrane damage.13
Acute Toxicity:
Oral
- LD50 values range from 639 mg/kg to 1646 mg/kg in rats depending on the chemical form of 2,4-D utilized in the study.3 Researchers found that 2,4-D was more toxic for mice, reporting an LD50 of 138 mg/kg.1 All chemical forms for 2,4-D are considered low in toxicity11 for acute oral exposure based on tests with rats.3 See the text boxes on Toxicity Classification and LD50/LC50.
Dermal
- Acute dermal LD50s ranged from 1829 mg/kg to greater than 2000 mg/kg in rabbits depending on the chemical form of 2,4-D. All chemical forms of 2,4-D are considered low in toxicity for acute dermal exposure based on studies using rabbits.3
- The acid and salt forms of 2,4-D are highly toxic to eye tissue, causing severe eye irritation. This is reflected in the signal word of the formulated product. The ester forms are not considered eye irritants, and have Iow to very low ocular toxicity.3
- The ester and salt forms of 2,4-D are considered slight skin irritants.3
LD50/LC50: A common measure of acute toxicity is the lethal dose (LD50) or lethal concentration (LC50) that causes death (resulting from a single or limited exposure) in 50 percent of the treated animals. LD50 is generally expressed as the dose in milligrams (mg) of chemical per kilogram (kg) of body weight. LC50 is often expressed as mg of chemical per volume (e.g., liter (L)) of medium (i.e., air or water) the organism is exposed to. Chemicals are considered highly toxic when the LD50/LC50 is small and practically non-toxic when the value is large. However, the LD50/LC50 does not reflect any effects from long-term exposure (i.e., cancer, birth defects or reproductive toxicity) that may occur at levels below those that cause death.
Inhalation
- All chemical forms of 2,4-D are of low to very low toxicity via inhalation based on studies using rats. Acute inhalation LC50s for rats ranged from 0.78 mg/L to greater than 5.4 mg/L depending on the chemical form.3 Most forms of 2,4-D are very low in toxicity, and the parent acid and TIPA salt forms are low in toxicity.3
Signs of Toxicity - Animals
- Dogs fed 2,4-D exhibited myotonia, vomiting, and weakness; dogs are more sensitive to chlorophenoxy acid herbicides than other animals.14 In addition, dogs and cats have displayed inappetance, anorexia, ataxia, salivation, diarrhea, lethargy, and convulsions following exposure to 2,4-D, which may include eating treated grass15 although the potential for this is unclear.16 Rats demonstrated incoordination, central nervous system depression and muscular weakness following acute oral dosing.3,17 Biochemical analysis of rat tissues suggested hepatic and muscle damage following acute, subchronic, and chronic oral exposures.17
Signs of Toxicity - Humans
- No occupational studies were found reporting signs or symptoms following exposure to 2,4-D under normal usage.
- Symptoms of acute oral exposure to 2,4-D include vomiting, diarrhea, headache, confusion, aggressive or bizarre behavior. A peculiar odor is sometimes noted on the breath. Skeletal muscle injury and renal failure may also occur.18 Systemic toxicity is mainly associated with suicide attempts.18
- Symptoms following dermal exposure may include irritation, and inhalation exposure may lead to coughing and burning sensations in the upper respiratory tract and chest.18 Prolonged exposure may result in dizziness.18 Chlorophenoxy compounds such as 2,4-D are quickly absorbed when swallowed, but absorption from dermal or inhalation exposure is low.13,18
- Case reports and observational studies provide the majority of information regarding the toxicological effects of 2,4-D in incidents involving human poisonings. Researchers compiled the medical cases of 69 people who ingested 2,4-D and other chlorophenoxy herbicides; 23 of these patients died.13 Ingestion led to vomiting, abdominal pain, diarrhea, and development of hypotension.13 Peripheral neuromuscular effects including muscle twitching, weakness, and loss of tendon reflexes have been reported.13 Neuromuscular effects have lasted several weeks to months and have been permanent in some cases.13
- Always follow label instructions and take steps to minimize exposure. If any exposure occurs, be sure to follow the First Aid instructions on the product label carefully. For additional treatment advice, contact the Poison Control Center at 1-800- 222-1222. If you wish to discuss an incident with the National Pesticide Information Center, please call 1-800-858-7378.
Chronic Toxicity:
Animals
- Subchronic oral exposure to 2,4-D caused damage to the eye, thyroid, kidney, adrenals, and the ovaries and testes of laboratory animals.3,19 A subchronic NOEL was established at 15 mg/kg/day based on studies in rats.19 See the text box on NOAEL, NOEL, LOAEL, and LOEL.
- The chronic toxicity NOEL in rats and mice was determined to be 5 mg/kg/day in two-year studies.12,20 The maximum tolerated dose in the two-year rat study was 150 mg/kg/day in male rats and 75 mg/kg/day in females.20 Additional NOEL and NOAEL doses were 15 mg/kg for rats in a 90-day study, and 1 mg/kg for dogs in a 12-month study, respectively.12,21 Rabbits exhibited toxicity following dosing with either acid, salt, or ester forms of 2,4-D at doses of 30 mg/kg/day or greater.4 Chronic NOAELs and LOELs in dogs, however, varied for different parameters studied and by chemical form.21
- Rats showed no outward signs of toxicity following exposure to 200 mg/L of 2,4-D in drinking water for 30 and 100 days, but biochemical analysis suggested hepatic and muscle damage.17
- Researchers fed rats 2,4-D at doses of 1, 15, 100, and 300 mg/kg/day acid equivalents (ae). Changes in blood and thyroid parameters, organ weight ratios, and body weight gain were noted at 100 and 300 mg/kg/day doses.19 Chronic toxicity in the eye, kidney, thyroid and liver of the rat were similar to effects found in subchronic studies.20 Eye lesions were associated only with high doses of 150 mg/kg/day.20
NOAEL: No Observable Adverse Effect Level
NOEL: No Observed Effect Level
LOAEL: Lowest Observable Adverse Effect Level
LOEL: Lowest Observed Effect Level
Humans
- No human data were found on chronic effects of 2,4-D other than epidemiological studies of cancer occurrence. Although pesticide use has been linked to Parkinson's disease and to respiratory disease in farmers, 2,4-D was not implicated in any relationships between pesticide exposure and subsequent disease.22,23 See the Carcinogenicity section below for more information on 2,4-D and cancer in humans. See the text box on Exposure.
Exposure: Effects of 2,4-D on human health and the environment depend on how much 2,4-D is present and the length and frequency of exposure. Effects also depend on the health of a person and/or certain environmental factors.
Endocrine Disruption:
- Because 2,4-D has demonstrated toxic effects on the thyroid and gonads following exposure, there is concern over potential endocrine-disrupting effects.3 2,4-D is included in the U.S. EPA June 2007 Draft List of Chemicals for Tier 1 Screening.24
Carcinogenicity:
Animals
- No oncogenic effects were observed in rats or mice following 2 years of dietary exposure of 2,4-D with concentrations ranging from 5-150 mg/kg/day or 5-300 mg/kg/day, respectively.20 Similarly, researchers did not observe immunotoxic or oncogenic responses in dogs dosed with 1.0-7.5 mg/kg/day for either 13 weeks or 1 year.21
- A case-control study in companion dogs concluded that there was a "modest association" between malignant lymphoma in the dogs and the use of 2,4-D in their owners' yards after accounting for other home and yard pesticide use.25 Other investigators have questioned the epidemiological association reported in that study.5,26
- Overall, there has been no consistent association between exposure to 2,4-D and tumor induction in animals.27 More recently, non-cytotoxic concentrations of 2,4-D were correlated to DNA damage and altered expression of some genes in hamster embryo cells.28
Humans
- The U.S. EPA evaluated 2,4-D for carcinogenic effects in 1988, 1992, and again in 2004. Each evaluation has concluded that "the data are not sufficient to conclude that there is a cause and effect relationship between exposure to 2,4-D and non- Hodgkin's Lymphoma." 2,4-D was categorized as "Group D - not classifiable as to human carcinogenicity" in 2004.3 See the text box on Cancer.
- The International Agency for Research on Cancer (IARC), had not assigned 2,4-D a cancer rating as of June 2008. However, in 1987, IARC placed the family of chlorophenoxy herbicides in Group 2B, possibly carcinogenic to humans.29
- A discussion of the history of classification decisions regarding the carcinogenicity of 2,4-D has been published. A confounding factor in determining the carcinogenicity of 2,4-D is the frequent simultaneous exposure of workers to 2,4-D in addition to 2,4,5-T and its contaminant TCDD (dioxin), or to other herbicides. However, other work examining incidents of exposure to 2,4-D without simultaneous exposure to 2,4,5-T has found some association between 2,4-D and non-Hodgkin's lymphoma.26
- Although the free acid form of 2,4-D did not damage chromosomes, there is limited evidence that commercial formulations may have the potential to do so.27 Overall, evidence for mutagenicity has been inconsistent.26,27,30
Cancer: Government agencies in the United States and abroad have developed programs to evaluate the potential for a chemical to cause cancer. Testing guidelines and classification systems vary. To learn more about the meaning of various cancer classification descriptors listed in this fact sheet, please visit the appropriate reference, or call NPIC.
Reproductive or Teratogenic Effects:
Animals
- Teratogenic effects were not observed in mice, rats, or rabbits unless the excretion capacity of the mother was overwhelmed following oral exposure to 2,4-D or its salt and ester forms.4,26 Reduced fetal viability was observed in hamsters following maternal dosing at 40 mg/kg/day during pregnancy, although effects did not follow a dose-response relationship.31
- Fetal abnormalities were observed in rats following oral doses of 90 mg/kg/day or greater beginning at fertilization; these doses were toxic to the mothers as well.4 A NOEL of 25 mg/kg/day was derived for fetal rats in one study, and a NOAEL of 12.5 mg/kg/day for the mothers and a developmental NOAEL of 50 mg/kg/day for the young were derived in another study.7 The overall maternal NOEL in rats was determined to be 8-17 mg/kg/day and overall developmental NOEL was 30 mg/kg/day 2,4-D acid equivalents.4
- Rabbit fetuses were unaffected at doses below 40 mg/kg/day administered to the dams although extra ribs were formed at doses above this threshold.4 In rabbits, the developmental NOEL was 30 mg/kg/day 2,4-D acid equivalents.4
Humans
- No experimental data are available regarding the effects of 2,4-D exposure on reproduction or development in humans. There are some reports of reproductive effects following occupational exposure to chlorophenoxy herbicides,7 including reduced sperm motility and viability following occupational exposure. Although motility and viability recovered over a period of several months, malformations were still present.32 Exposure to multiple pesticides in epidemiological studies make inference difficult.26
Fate in the Body:
Absorption
- The greatest absorption rates in humans are from oral exposure, with much less absorption occurring following dermal or inhalation exposures.18 Absorption rates following ingestion are dose-dependent in laboratory animals, with larger doses persisting in the gastrointestinal tract for longer periods of time.7 In humans, plasma levels following 5 mg/kg oral ingestion peaked between 4-24 hours post-exposure.7
- Dermal exposure is considered the most likely route of exposure during product use.7 Absorption of 2,4-D across the skin occurs more slowly and is less complete, and varies by chemical form, product formulation, species, and site of application.7 Dermal absorption may be increased significantly with application of some sunscreens, insect repellents, or by alcohol consumption, as demonstrated in laboratory studies using rats and mice.33,34,35 Hairless mouse skin absorbed 39% of a 100 μL dose in 24 hours.34
Distribution
- In laboratory animals, the primary target organs for 2,4-D toxicity were the eye, thyroid, kidney, adrenal glands, and ovaries or testes following subchronic oral exposure at doses above the threshold of saturation for renal clearance.3 Biochemical changes suggested that liver and muscle damage occurred in rats at acute, subchronic, and chronic doses.17
- In humans, 2,4-D has a wide volume of distribution due to its water solubility, but it does not accumulate in any tissue.7
Metabolism
- Metabolism of 2,4-D is minimal in humans, with nearly all of it excreted unchanged as the parent compound.7,36 The remainder is excreted as an unspecified 2,4-D conjugate.37
- In animals, little 2,4-D is metabolized prior to excretion. Up to 3.2% of the applied dose in rats was excreted as an unspecified polar metabolite.26 In sheep and cattle, muscle, liver, kidney, and fat tissue contained the metabolite 4-chlorocatechol. 38 Dogs must metabolize the parent compound prior to excretion, due to their reduced ability to excrete organic acids.39
- No reactive intermediate metabolic products for 2,4-D have been identified in any species.26
Excretion
- In humans, 2,4-D is rapidly excreted from the body, primarily in the urine.7 Much of the compound appears to be eliminated unchanged, although some 2,4-D is eliminated from the body as a conjugate.37 The percent of original dose excreted as a polar, acid-hydrolyzable metabolite was 4.8-27.0%.26 The elimination half-life from blood plasma in humans orally dosed with 5 mg/kg of 2,4-D was 11.6 hours.37 These human volunteers excreted more than 75% of 2,4-D in their urine within 96 hours of oral dosing.36 Concentrations in blood plasma paralleled concentrations excreted in urine.36 Some 2,4-D may be excreted in perspiration but this process appears to occur more slowly compared with urinary excretion.7
- Excretion of 2,4-D in animals depends on the species, formulation, and dose.40 In rats, elimination of orally administered doses of 5 and 50 mg/kg 2,4-D took 24 hours, and the urine was composed almost entirely of unmetabolized 2,4-D.39
- Dogs excreted a 5 mg/kg oral dose primarily in their urine with minor amounts detected in feces.39 Dogs dosed with 50 mg/kg excreted equal amounts in urine and feces and excretion was incomplete at 120 hours post-dose.39 Because dogs appear to be deficient in their ability to excrete organic acids, 2,4-D must be metabolized prior to excretion.39 Dogs orally dosed with 2,4-D excreted the parent compound, several conjugates and one unidentified compound in their urine.39
- Excretion of 2,4-D in urine is dose-dependent but nonlinear, with percent excreted in urine declining at higher doses.7 In all of the species of animals studied, 2,4-D is excreted quickly and almost entirely in the urine.7
Medical Tests and Monitoring:
- Biomarkers of exposure to 2,4-D have been reported in the scientific literature.41 Scientists used high-performance liquid chromatography with tandem mass spectrometry to detect 2,4-D in urine.41,42
- Laboratory testing for 2,4-D is not widely available to physicians.
- 2,4-D was detected at low concentrations in urine samples collected from all age groups in a large study of the American public.41 However, how these residues may affect human health is presently not clear,41 and the relationship between exposure level and biomarker is unknown.43
Environmental Fate:
Soil
- 2,4-D amine salts and esters are not persistent under most environmental conditions.3 Typically, the ester and amine forms of 2,4-D are expected to degrade rapidly to the acid form.3 Soil half-life values have been estimated at 10 days for the acid, diethylamine salt, and ester forms.44 Another study estimated a soil half-life for the ester form EHE ranging from 1-14 days with a median half-life of 2.9 days.3 In aerobic mineral soils, a half-life of 6.2 days was estimated.3 A granular formulation of the BEE form was detected in aquatic sediments for 186 days post-application, perhaps due to either the formulation or slow de-esterification of the sediment-bound chemical.3 See the text box on Half-life.
- Microbial degradation of 2,4-D in soil involves hydroxylation, cleavage of the acid side-chain, decarboxylation, and ring opening.1 The ethyl hexyl form of the compound is rapidly hydrolyzed in soil and water to form the 2,4-D acid.1 Other comparative studies demonstrated that ester and amine salt forms of 2,4-D have similar soil dissipation rates because they are converted rapidly to the same anionic form.45
- 2,4-D has a low binding affinity in mineral soils and sediment, and in those conditions is considered intermediately to highly mobile.3 In sandy loam, sand, silty clay loam, and loam soil, Koc values of 70, 76, 59, and 117 mL/g, respectively, were obtained,3 indicating low binding affinity in these soil types. Although 2,4-D is highly mobile, rapid mineralization rates may reduce the potential of 2,4-D to affect groundwater.46 Microbes may play a major role in degradation.2
- Break-down products of 2,4-D detected in laboratory experiments included 1,2,4-benzenetriol, 2,4-dichlorophenol (2,4- DCP), 2,4-dichloroanisole (2,4-DCA), 4-chlorphenol, chlorohydroquinone (CHQ), volatile organics, bound residues, and carbon dioxide. These degradates are expected to be of low occurrence in the environment, of low toxicity, or both.3
The "half-life" is the time required for half of the compound to break down in the environment.
1 half-life = 50% remaining
2 half-lives = 25% remaining
3 half-lives = 12% remaining
4 half-lives = 6% remaining
5 half-lives = 3% remaining
Half-lives can vary widely based on environmental factors. The amount of chemical remaining after a half-life will always depend on the amount of the chemical originally applied. It should be noted that some chemicals may degrade into compounds of toxicological significance.
Water
- The half-life of 2,4-D in aerobic aquatic environments was estimated to be 15 days and in anaerobic aquatic laboratory studies, 41-333 days.3 A granular formulation of the BEE form degraded rapidly in the water column in alkaline conditions but was present in sediments for 186 days.3
- The ethyl hexyl form is rapidly hydrolyzed in water to 2,4-D acid, with a degradation half-life (DT50) of less than one day.1 Ester forms of 2,4-D hydrolyze at rates that are pH dependent; the hydrolysis half-life of the butoxy ester increased from 9 hours at pH 8 to more than one year in more acidic conditions with a pH of 5.38 The acid form of 2,4-D is very resistant to abiotic hydrolysis.3
- 2,4-D has been detected in streams and shallow groundwater at low concentrations, in both rural and urban areas.3,47,48
Air
- Volatility for most forms of 2,4-D is low (see the table on 2,4-D and associated forms). However, the vapor pressure of some ester forms range from 1.1 x 10-3 to 2.3 x 10-3 mmHg,2 indicating that these forms readily volatilize. The Henry's Law Constant for 2,4-D acid is 3.5 x 10-4 at pH 7,49 indicating low potential for movement from water to air.
- No data were found regarding the degradation of 2,4-D in the atmosphere.
Plants
- The ester forms of 2,4-D penetrate foliage, whereas plant roots absorb the salt forms.12 Ester forms are converted to the acid within the plant, then accumulate in cells due to passive diffusion down the concentration gradient.12 Active transport within the plant may also occur.12 Accumulation occurs primarily at the meristem tissue of roots and shoots.1
- Forest dissipation studies indicated that the ethyl hexyl ester form of 2,4-D degraded slowly on foliage and in leaf litter.3 Residues of an ester form of 2,4-D were detected in samples of dead birch leaves for up to three years post-application.50
Indoor
- No data were available on indoor persistence.
Food Residue
- 2,4-D was not included in the list of pesticides detectable in regulatory monitoring.51
- Traces of 2,4-D were detected in 49.3% of finished drinking water samples and 53.7% of untreated water samples (365 and 367 samples taken, respectively), with detections between 1.1 and 2416.0 parts per trillion (ppt). These concentrations are well below the maximum contaminant level (MCL) of 70,000 ppt set by the U.S. EPA for finished drinking water.52 In bottled water, only 2 of 367 samples contained 2,4-D, with residues of 3.2 and 4.2 ppt.52 See the text box on Maximum Contaminant Level (MCL).
Maximum Contaminant Level (MCL): The MCL is the highest level of contaminant that is legally allowed in drinking water. The MCL is enforceable. The MCL is typically measured in milligrams (mg) of contaminant per liter (L) of water.
U.S. Environmental Protection Agency, National Primary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#one
Ecotoxicity Studies:
Birds
- LD50 values range from 472 mg/kg for acute oral exposure in pheasants, to 668 mg/kg in pigeons and Japanese quail, to greater than 1000 mg/kg in wild ducks.1 The acute oral LD50 for the dimethyl amine salt form of the compound was 500 mg/kg for bobwhite quail, and the acute oral LD50 for the ethyl hexyl form was 663 mg/kg in mallard ducks. The acute oral LD50 for wild ducks was in excess of 2025 mg/kg for the sodium salt form of 2,4-D.1 Overall, 2,4-D is moderately toxic to practically non-toxic to birds. There are no pronounced differences in toxicity based on the form of 2,4-D.3
- Five-day studies estimated LC50 values for bobwhite quail and mallard ducks at greater than 5620 ppm.1 Chronic studies have also demonstrated low toxicity, with no effects observed below very high exposure levels such as concentrations in drinking water greater than the solubility of the chemical.2 Under field conditions, eggs of ground-nesting birds could be exposed, but eggshell permeability to 2,4-D is low and treating eggshells with high concentrations of 2,4-D did not reduce hatchability or cause chick abnormalities.2
Fish and Aquatic Life
- Toxicity to fish and aquatic invertebrates varies widely depending on chemical form, with esters being the most toxic.1,2 Acid and amine salt LC50s range from greater than 80 to 2244 mg acid equivalents per liter (mg ae/L) whereas the esters range from less than 1.0 to 14.5 mg acid equivalents per liter.3 The greater toxicity generally of the esters in fish is likely due to the greater absorption rates of the esters through the gills, where they are hydrolyzed to the acid form.2 The acute LC50 of the dimethyl amine salt form to rainbow trout was 100 mg/L,1 which is considered slightly toxic.
- The acute LC50 of the ethyl hexyl form to rainbow trout was greater than its solubility in water.1 The LD50 value for the isoctyl form (CASRN 25168-26-7) in cutthroat trout was 0.5-1.2 mg/L,1 or moderately to highly toxic. Adult fathead minnows exhibited toxic effects at chronic exposures of the butoxyl ethanol ester form that were 1/10 to 1/45 of the 96-hour LC50 concentrations.2 Early life stages of fish are more susceptible compared with adult fish or eggs.2
- Daphnia exposed to the acid form for 21 days exhibited an LC50 of 235 mg/L when exposed to 2,4-D acid for 21 days, and an LC50 of 5.2 mg/L when exposed to the ethyl hexyl form for 48 hours.1 Therefore, the acid form is practically non-toxic to Daphnia but the ethyl hexyl form is moderately toxic. As with fish, esters are more toxic than acid or amine salt forms to freshwater aquatic invertebrates, with LC50 values ranging from 25 to 643 mg ae/L for the acid and amine salt forms but 2.2 to 11.8 mg ae/L for esters.3 The relative toxicities for acids and salts are slightly toxic to practically non-toxic, whereas the esters are moderately to slightly toxic.
- Marine invertebrate sensitivities are similar to aquatic invertebrates, with LC50 values of 50-830 mg ae/L for acid and salt forms and >0.092 to >66 mg ae/L for ester forms.3 The corresponding relative toxicity values are slightly toxic to practically non-toxic for the salts and acid but highly toxic to practically non-toxic for the ester forms.
- Researchers have estimated a No Observed Effect Concentration (NOEC) of 16.1 mg ae/L for the DEA ester and 79.0 mg ae/L for the acid form based on survival and reproduction for DEA and number of young produced for the acid form. The freshwater aquatic invertebrate NOEC for the BEE ester was estimated at 0.2 mg ae/L based on survival and reproduction.3
- 2,4-D is marketed for controlling aquatic plants. Therefore, the lethal concentrations are reported as effective concentrations for killing half the target population (EC50). Researchers estimated an EC50 of 0.58 mg/L for duckweed (Lemna gibba). A variety of algal species exhibited LC50 values ranging between 0.23 and greater than 30 mg/L for the ethyl hexyl form.1 The EC50 for the dimethyl amine salt form against Selenastrum capricornutum was estimated at 51.2 mg/L.1 No effects were recorded for 19 genera of algae exposed to 2,4-D at concentrations of up to 222 mg/L.2 However, the ester forms were toxic to some algae at much lower concentrations.2 See the text box on EC50.
- A mesocosm study indicated that an unspecified form of 2,4-D applied at 0.117 mL/m2 had no negative effects on species richness, biomass, or survival on algae and 25 species of aquatic animals, including frog larvae, salamanders, snails, and a range of other invertebrates.53 Ninety-six-hour LC50 concentrations for several species of amphibian larvae exceeded 100 mg/L for the amine salt forms.2 2,4-D acid, 2,4-D EHE, and 2,4-D DMA are considered practically non-toxic to amphibian larvae based on tests with Rana pipiens.3
- Bioavailability and uptake of 2,4-D by organisms are strongly influenced by pH, temperature, and other environmental factors.2 The sensitivity of aquatic invertebrates to 2,4-D increases with temperature; concentrations below those associated with short-term toxic effects impaired reproduction when ambient temperature was elevated.2 Although some aquatic invertebrates appear to sense and avoid 2,4-D in the water, others do not, even when exposed to lethal concentrations.2 Fish appear to avoid 2,4-D in a dose-dependent manner until the onset of toxic effects.2 Toxicity of 2,4-D was increased when fish were simultaneously exposed to 2,4-D and carbaryl or picloram.2
EC50: The median effective concentration (EC50) may be reported for sublethal or ambiguously lethal effects. This measure is used in tests involving species such as aquatic invertebrates where death may be difficult to determine. This term is also used if sublethal events are being monitored.
Newman, M.C.; Unger, M.A. Fundamentals of Ecotoxicology; CRC Press, LLC.: Boca Raton, FL, 2003; p 178.
Terrestrial Invertebrates
- LC50 values for 24-hour exposures in honey bees (Apis mellifera) were estimated to be 104 and 115 μg per bee. Researchers estimated the LD50 at greater than 10 μg/bee, so 2,4-D is considered practically non-toxic.3 Effects on bee longevity varied according to dose and 2,4-D form.2
- 2,4-D is not considered hazardous to beneficial insects due to its low insecticidal activity and an adequate safety margin when products containing 2,4-D are used at recommended levels.2,3
- Carabid beetles (Carabidae) exposed to sand dosed with 1 g/m2 exhibited greater than 50% mortality after 4 days.2
- The calculated 48-hour LC50 concentration for earthworms (Lumbricus rubellus) exposed to filter paper treated with 2,4-D was 61.6 μg/cm.22
- Effects of 2,4-D on soil microorganisms were species-dependent.2
Regulatory Guidelines:
- The reference dose (RfD) for 2,4-D is 0.01 mg/kg/day.54 See the text box on Reference Dose (RfD).
- The U.S. EPA has classified 2,4-D as "Group D - not classifiable with regard to human carcinogenicity" in 2004.3 IARC had not assigned 2,4-D a cancer rating as of December 2007. However, the chlorophenoxy herbicides as a group were classified in Group 2B, meaning that they are considered to be possibly carcinogenic to humans, by IARC in 1987.29 See the text box on Cancer.
- The threshold limit value, or TLV, for 2,4-D is 10 mg/m3 for an 8-hour time weighted average exposure.55 This limit is based on results of animal feeding experiments.43 This same dose was selected by the Occupational Safety and Health Administration (OSHA) for the permissible exposure limit (PEL) for an 8-hour time weighted average exposure and by the National Institute for Occupational Safety and Health (NIOSH) for the recommended exposure limit (REL) for a 10-hour workday and a 40-hour workweek.43
- The MCL for 2,4-D in drinking water is 0.07 mg/L.56 See the text box on Maximum Contaminant Level (MCL).
Reference Dose (RfD): The RfD is an estimate of the quantity of chemical that a person could be exposed to every day for the rest of their life with no appreciable risk of adverse health effects. The reference dose is typically measured in milligrams (mg) of chemical per kilogram (kg) of body weight per day.
U.S. Environmental Protection Agency, Integrated Risk Information System, IRIS Glossary, 2009. https://www.epa.gov/iris/iris-glossary#r
Please cite as: Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. 2008. 2,4-D Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. https://npic.orst.edu/factsheets/archive/2,4-DTech.html.
References:
- Tomlin, C. D. S. The Pesticide Manual: A World Compendium, 14th ed.; British Crop Protection Council: Surrey, UK, 2006.
- WHO. Environmental Health Criteria 84, Environmental Aspects - 2,4-Dichlorophenoxyacetic acid (2,4-D); International Programme on Chemical Safety, World Health Organization: Geneva, Switzerland, 1989.
- Reregistration Eligibility Decision (RED) 2,4-D; EPA 738-R-05-002; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2005.
- Charles, J. M.; Hanley, T. R.; Wilson, R. D.; Van Ravenzwaay, B.; Bus, J. S. Developmental Toxicity Studies in Rats and Rabbits on 2,4-Dichlorophenoxyacetic Acid and its Forms. Toxicol. Sci. 2001, 60, 121-131.
- Carlo, G. L.; Cole, P.; MIller, A. B.; Munro, I. C.; Solomon, K. R.; Squire, R. A. Review of a Study Reporting an Association between 2,4-Dichlorophenoxyacetic Acid and Canine Malignant Lymphoma: Report of an Expert Panel. Regul. Toxicol. Pharmacol. 1992, 16, 245-252.
- Kamrin, M. A. Pesticide Profiles: Toxicity, Environmental Impact, and Fate; Lewis Publishers: New York, 1997, p 306.
- Munro, I. C.; Carlo, G. L.; Orr, J. C.; Sund, K. G.; Wilson, R. M.; Kennepohl, E.; Lynch, B. S.; Jablinske, M.; Lee, N. L. A Comprehensive, Integrated Review and Evaluation of the Scientific Evidence Relating to the Safety of the Herbicide 2,4-D. J. Am. Coll. Toxicol. 1992, 11, (5), 559-664.
- FAO. Pesticide Residues in Food - Evaluations Part 1: Residues; FAO Plant Production and Protection Paper 152/1, Food and Agriculture Organization of the United Nations and World Health Organization: Rome, 1988; Vol. 1, pp 179-189.
- Hazardous Substances Databank (HSDB), 2,4-D; U.S. Department of Health and Human Services, National Institutes of Health, National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/202 (accessed June 2008), updated June 2005.
- Pesticide Products. Pest-Bank [CD-ROM] 2007.
- Label Review Manual; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs. https://www.epa.gov/sites/default/files/2018-04/documents/chap-07-mar-2018.pdf (accessed June 2008), updated Sept 2007.
- Herbicide Handbook, 8th ed.; Vencill, W.K. Ed.; Weed Science Society of America: Lawrence, KS, 2002; pp 113-115.
- Bradberry, S. M.; Proudfoot, A. T.; Vale, J. A. Poisoning Due to Chlorophenoxy Herbicides. Toxicol. Rev. 2004, 23 (2), 65-73.
- Peterson, M. E.; Talcott, P. A. Small Animal Toxicology, 2nd ed.; Saunders Elsevier: St. Louis, 2006; pp 734-735.
- Campbell, A.; Chapman, M. Handbook of Poisoning in Dogs and Cats; Blackwell Science Ltd.: Oxford, England, 2000; pp 220-221.
- Arnold, E. K.; Lovell, R. A.; Beasley, V. R.; Parker, A. J.; Stedelin, J.R. 2,4-D Toxicosis III: An Attempt to Produce 2,4-D Toxicosis in Dogs on Treated Grass Plots. Vet. Hum. Toxicol. 1991, 33 (5), 457-461.
- Paulino, C. A.; Guerra, J. L.; Oliveira, G. H.; Palmero-Neto, J. Acute, Subchronic and Chronic 2,4-Dichlorophenoxyacetic Acid (2,4-D) Intoxication in Rats. Vet. Hum. Toxicol. 1996, 38 (5), 348-352.
- Reigart, J. R.; Roberts, J. R. Chlorophenoxy Herbicides. Recognition and Management of Pesticide Poisonings, 5th ed.; U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 1999; pp 94-96.
- Charles, J. M.; Cunny, H. C.; Wilson, R. D.; Bus, J. S. Comparative Subchronic Studies on 2,4-Dichlorophenoxyacetic Acid, Amine, and Ester in Rats. Fundam. Appl. Toxicol. 1996, 33, 161-165.
- Charles, J. M.; Bond, D. M.; Jeffries, T. K.; Yano, B. L.; Stott, W. T.; Johnson, K. A.; Cunny, H. C.; Wilson, R. D.; Bus, J. S. Chronic Dietary Toxicity/ Oncogenicity Studies on 2,4-Dichlorphenoxyacetic Acid in Rodents. Fundam. Appl. Toxicol. 1996, 33, 166-172.
- Charles, J. M.; Dalgard, D. W.; Cunny, H. C.; Wilson, R. D.; Bus, J. S. Comparative Subchronic and Chronic Dietary Toxicity Studies on 2,4-Dichlorophenoxyacetic Acid, Amine, and Ester in the Dog. Fundam. Appl. Toxicol. 1996, 29, 78-85.
- Hoppin, J. A.; Umbach, D. M.; London, S. J.; Alavanja, M. C. R.; Sandler, D. P. Chemical Predictors of Wheeze among Farmer Pesticide Applicators in the Agricultural Health Study. Am. J. Respir. Crit. Care Med. 2002, 165, 683-689.
- Kamel, F.; Tanner, C. M.; Umbach, D. M.; Hoppin, J. A.; Alavanja, M. C. R.; Blair, A.; Comyns, K.; Goldman, S. M.; Korell, M.; Langston, J. W.; Ross, G. W.; Sandler, D. P. Pesticide Exposure and Self-reported Parkinson's Disease in the Agricultural Health Study. Am. J. Epidemiol. 2006, 165 (4), 364-374.
- Draft List of Initial Pesticide Active Ingredients and Pesticide Inerts to be Considered for Screening Under the Federal Food, Drug, and Cosmetic Act. Fed. Regist. June 18, 2007, 72 (116), 33486-33503.
- Hayes, H. M.; Tarone, R. E.; Cantor, K. P.; Jessen, C. R.; McCurnin, 25. D. M.; Richardson, R. C. Case-Control Study of Canine Malignant Lymphoma: Positive Association With Dog Owner's Use of 2,4-Dichlorphenoxyacetic Acid Herbicides. J. Natl. Cancer Inst. 1991, 83, 1226-1231.
- Garabant, D. H.; Philbert, M. A. Review of 2,4-Dichlorophenoxyacetic Acid (2,4-D) Epidemiology and Toxicology. Crit. Rev. Toxicol. 2002, 32 (4), 233-257.
- Gandhi, R.; Wandji, S.-A.; Snedeker, S. Critical Evaluation of Cancer Risks from 2,4-D. Rev. Environ. Contam. Toxicol. 2000, 167, 1-33.
- Maire, M. A.; Rast, C.; Landkocz, Y.; Vasseur, P. 2,4-Dichlorophenoxyacetic Acid: Effects on Syrian Hamster Embryo (SHE) Cell Transformation, c-Myc Expression, DNA Damage, and Apoptosis. Mutat. Res. 2007, 631, 124-136.
- IARC Monographs on the Evaluation of Carcinogenicity Risks to Humans. Overall Evaluations of Carcinogencity: An Updating of IARC Monographs, Volumes 1 to 42; International Agency for Research on Cancer: Lyon, France, 1987; Supplement 7.
- Madrigal-Bujaidar, E.; Hernandez-Ceruelos, A.; Chamorro, G. Induction of sister chromatid exchanges by 2,4-dichlorophenoxyacetic acid in somatic and germ cells of mice exposed in vivo. Food Chem. Toxicol. 2001, 39, 941-946.
- Collins, T. F. X.; Williams, C. H. Teratogenic Studies with 2,4,5-T and 2,4-D in the Hamster. Bull. Environ. Contam. Toxicol. 1971, 6 (6), 559-567.
- Lerda, D.; Rizzi, R. Study of reproductive function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid (2,4-D). Mutat. Res. 1991, 6 (6), 1, 47-50.
- Brand, R. M.; McMahon, L.; Jendrzejewski, J. L.; Charron, A. R. Transdermal absorption of the herbicide 2,4-dichlorphenoxyacetic acid is enhanced by both ethanol consumption and sunscreen application. Food Chem. Toxicol. 2007, 456, 93-97.
- Brand, R. M.; Spaulding, M.; Mueller, C. Sunscreens Can Increase Dermal Penetration of 2,4-Dichlorphenoxyacetic Acid. J. Toxicol. Clin. Toxicol. 2002, 40 (7), 827-832.
- Pont, A. R.; Charron, A. R.; Brand, R. M. Active ingredients in sunscreens act as topical penetration enhancers for the herbicide 2,4-dicholorphenoxyacetic acid. Toxicol. Appl. Pharmacol. 2004, 195, 348-354.
- Kohli, J. D.; Khanna, R. N.; Gupta, B. N.; MDhar, M. M.; Tandon, J. S.; Sircar, K. P. Absorption and Excretion of 2,4-Dichlorophenoxyacetic Acid in Man. Xenobiotica 1974, 4 (2), 97-100.
- Sauerhoff, M. W.; Braun, W. H.; Blau, G. E.; Gehring, P. J. The Fate of 2,4-Dichlorophenoxyacetic Acid (2,4-D) Following Oral Administration to Man. Toxicol. 1977, 8, 3-11.
- Roberts, T. R. Metabolic Pathways of Agrochemicals - Part 1: Herbicides and Plant Growth Regulators; The Royal Society of Chemistry: Cambridge, UK, 1998; pp 66-74.
- Van Ravenzwaay, B.; Hardwick, T. D.; Needham, D.; Pethen, S.; Lappin, G. J. Comparative metabolism of 2,4-dichlorophenoxyacetic acid (2,4-D) in rat and dog. Xenobiotica 2003, 33 (8), 805-821.
- Arnold, E. K.; Beasley, V. R. The Pharmacokinetics of Chlorinated Phenoxy Acid Herbicides: A Literature Review. Vet. Hum. Toxicol. 1989, 31 (12), 121-125.
- CDC. Third National Report on Human Exposure to Environmental Chemicals; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, 2005; pp 390-394.
- Olsson, A. O.; Baker, S. E.; Nguyen, J. V.; Romanoff, L. C.; Udunka, S. O.; Walker, R. D.; Flemmen, K. L.; Barr, D. B. A liquid chromatographytandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and DEET in human urine. Anal. Chem. 2004, 76 (9), 2453-2461.
- OSHA. Occupational Safety and Health Guideline for 2,4-D (Dichlorophenoxyacetic Acid); U.S. Department of Labor, Occupational Safety and Health Administration. https://www.osha.gov/chemicaldata/750 (accessed May 2008), updated April 1999.
- Vogue, P.A.; Kerle, E.A.; Jenkins, J.J. OSU Extension Pesticide Properties Database; Oregon State University: Corvallis, OR, 2004.
- Wilson, R. D.; Geronimo, J.; Armbruster, J. A. 2,4-D Dissipation in Field Soils After Applications of 2,4-Dimethylamine Salt and 2,4-D Ethylhexyl Ester. Environ. Toxicol. Chem. 1997, 16 (6), 1239-1246.
- Boivin, A.; Amellal, S.; Schiavon, M.; van Genuchten, M. T. 2,4-dichlorophenoxyacetic acid (2,4-D) sorption an ddegradation dynamics in three agricultural soils. Environ. Pollut. 2005, 138, 92-99.
- Pesticides in Surface and Groundwater of the United States: Summary of Results of the National Water Quality Assessment Program (NAWQA); U.S. Geological Survey: Reston, VA, 1998.
- McPherson, A. K.; Moreland, R. S.; Atkins, J. B. Occurrence and Distribution of Nutrients, Suspended Sediment, and Pesticides in the Mobile River Basin, Alabama, Georgia, Mississippi, and Tennessee, 1999-2001; Water-Resources Investigations Report 03- 4203, U.S. Geological Survey: Montgomery, AL, 2003; pp 1-2, 44, 57.
- Rice, C. P.; Chernyak, S. M.; McConnell, L. L. Henry's Law Constants for Pesticides Measured as a Function of Temperature and Salinity. J. Agric. Chem. 1997, 45, 2291-2298.
- Torstensson, N. T. L.; Lundgren, L. N.; Stenstrom, J. Influence of Climatic and Edaphic Factors on Persistence of Glyphosate and 2,4-D in Forest Soils. Ecotoxicol. Environ. Saf. 1989, 18, 230-239.
- Food and Drug Administration Pesticide Program Residue Monitoring 2003; U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Plant and Dairy Foods: Washington, DC, 1993-2003.
- Pesticide Data Program Annual Summary, Calendar Year 2006; U.S. Department of Agriculture, Agricultural Marketing Service: Washington, DC, 2007.
- Relyea, R. A. The Impact of Insecticides and Herbicides on the Biodiversity and Productivity of Aquatic Communities. Ecolo. Appl. 2005, 15 (2), 618-627.
- Intergrated Risk Information System, 2,4-Dichlorphenoxyacetic Acid (2,4-D) (CASRN 94-75-7); U.S. Environmental Protection Agency. https://epa.gov/ncea/iris/subst/0150.htm (assessed April 2008), updated Jan 2008.
- ACGIH. TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; American Conference of Governmental Industrial Hygienists Worldwide: Cincinnati, 2003; p 24.
- Drinking Water Contaminants; U.S. Environmental Protection Agency. https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants (accessed May 2008), updated Feb 2008.

